Minocycline-loaded nHAP/PLGA microspheres for prevention of injury-related corneal angiogenesis | Journal of Nanobiotechnology
Characterization of nHAP, nHAP/PLGA, and MINO@PLGA
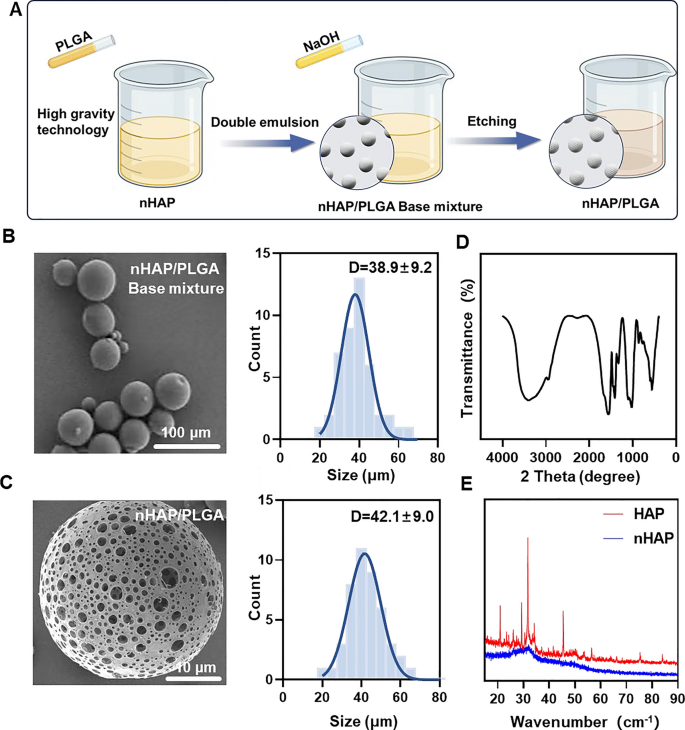
MINO was loaded onto porous nHAP/PLGA microspheres in a simple, efficient, cost-effective, and environmentally-friendly manner to synthesize MINO@PLGA. It functions by enhancing TGF-β expression, reducing ocular inflammation, accelerating corneal epithelial healing, and inhibiting neovascularization (Graphical Abstract). Initially, a nano-hydroxyapatite (nHAP) with ultra-small size and high dispersion stability is prepared using ultracentrifugation technology followed by combining it with PLGA to form a base mixture. This mixture is then etched with NaOH to produce the final nHAP/PLGA microspheres (Fig. 1A). SEM reveals that nHAP/PLGA base mixture has a good dispersibility and spherical shape with particle size of 38.9 ± 9.2 μm (Fig. 1B). In contrast, the etched nHAP/PLGA microspheres exhibit a porous structure suitable for loading MINO with particle size of 42.1 ± 9.0 μm (Fig. 1C). The FT-IR spectrum of nHAP (Fig. 1D), displays peaks for O–H at 3300 cm−1 to 3400 cm−1 and 1500 cm−1 to 1600 cm−1. Peaks for P-O are evident at 1000 cm−1 to 1100 cm−1 and 500 cm−1 to 600 cm−1. This indicates that the product is hydroxyapatite. X-ray diffraction (XRD) indicates that nHAP has lower diffraction peaks compared to HAP, suggesting enhanced crystalline stability in the synthesized nHAP (Fig. 1E).
Incorporating nHAP retains the scaffold’s biocompatibility and improves the biodegradability of the microspheres. Moreover, the XRD of nHAP displays an amorphous structure, indicating enhanced drug loading and sustained-release capabilities. The drug-loading capacity of nHAP/PLGA microspheres, measured at an absorbance of 360 nm, is approximately 50%. Biodegradable polymers have garnered increasing interest due to their biocompatibility, non-toxicity, and diverse properties. PLGA stands out as one of the most widely recognized biodegradable polymers. Notably, it has received approval from the FDA for applications in drug delivery, diagnostics, and various fields of clinical and basic science research, encompassing cardiovascular disease, cancer, vaccines, and tissue engineering [17]. An in vivo ocular anti-inflammatory study conducted in rabbit eyes confirmed the superior efficacy of drug-loaded nanoparticles in comparison to drug solutions [18]. Two research teams have also designed PLGA nanoparticles loaded with tacrolimus for topical ocular instillations [19]. Alshamsan et al. developed PLGA-tacrolimus nanoparticles using the emulsification-diffusion method, demonstrating a significant enhancement in ocular bioavailability through the entrapment of tacrolimus by nanoparticles [20]. Similarly, Benita et al. developed PLGA-tacrolimus nanoparticles using a well-established solvent displacement method. It’s worth noting that repeated ocular instillations of these nanoparticles in rat eyes resulted in elevated tacrolimus levels within the eye, while plasma concentrations remained low [19].
Furthermore, the size of micro-particles plays a crucial role in the drug release rate. A reduction in particle size leads to an increase in surface area and mass transfer for a fixed mass of drug and polymer [21]. Lyu et al. [9] encapsulated bevacizumab (BEV) within the pores of mesoporous silica nanoparticles (MSNs), forming BEV@MSN nanoparticles with an average diameter of 39.3 ± 5.3 nm. Meanwhile, Zhang et al. [22] prepared PLGA-bevacizumab nanoparticles with a hydrodynamic diameter of approximately 133 nm, achieving an entrapment efficiency and loading efficiency of around 80.0% and 6.8%, respectively. In this study, the loading of MINO into porous nHAP/PLGA microspheres, with a drug loading capacity of approximately 50%, could enhance the bioavailability and augment the anti-angiogenic efficiency of MINO. Moreover, MINO@PLGA nanoparticles exhibit a spherical shape with a smooth surface, which could minimize susceptibility to shear forces and facilitate effective interactions with cell surfaces, thereby increasing cellular uptake. Consequently, PLGA-mediated MINO delivery promises to extend the residence time of MINO in vivo, leading to sustained concentrations and improved drug bioavailability.
MINO@PLGA microspheres release, degradation, permeation and inhibit cell migration
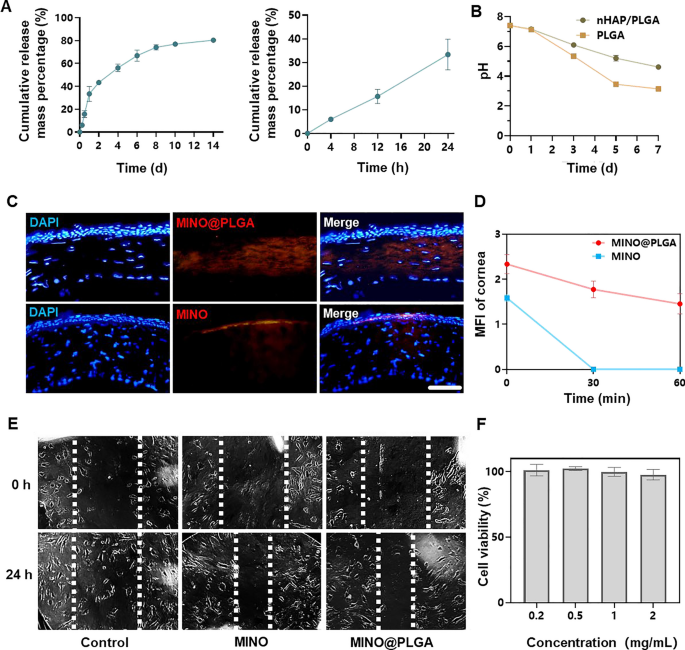
The cumulative release of MINO from PLGA microspheres increased over time, reaching approximately 80% by day 14 (Fig. 2A). This sustained release pattern suggests that the microspheres provide a prolonged therapeutic effect, potentially countering the activity of corneal neovascularization over an extended period. The pH of PLGA microspheres declined within 7 days, indicating the degradation of PLGA microspheres might result in an acidic environment (Fig. 2B). However, due to the irritant nature of low pH on eyes, there are limitations to the use of PLGA microspheres. In contrast, nHAP/PLGA demonstrated a more stable pH post-degradation compared to PLGA, suggesting better tissue compatibility. Combined images revealed that MINO@PLGA effectively remained within the corneal epithelium, whereas standalone MINO failed to penetrate the corneal epithelium (Fig. 2C). This indicates that MINO@PLGA enhances drug contact time with ocular tissues, providing a controlled and protected delivery of drugs, potentially extending therapeutic effects. Pharmacokinetic data derived from fluorescence intensity measurements of the cornea (Fig. 2D) provide the sustained release profile of our formulation. At the 60-min mark, a significant difference in fluorescence intensity was observed between the MINO and MINO@PLGA groups. This difference indicates that the MINO@PLGA microspheres not only facilitate enhanced corneal penetration but also contribute to a prolonged drug contact time with ocular tissues. Specifically, while the average retention time of standard ocular medications on the eye surface is 2–3 min [23], our MINO@PLGA formulation exhibited prolonged residence time. Such data underscore the efficacy of the MINO@PLGA system in maintaining sustained drug concentrations at the target site, thus potentially extending therapeutic effects.
Minocycline microspheres release, degradation, permeation and inhibit cell migration. A Minocycline microspheres release profile. B Degradation of nHAP/PLGA over time with pH. C Fluorescent retention observation of e minocycline or MINO@PLGA in cornea (scale bar: 100 μm). D Changes in the cornea average fluorescence intensity of drug after eye drop at 0, 30, and 60 min (n = 3). E Scratch healing assay of HUVECs treated with minocycline (1 mg/mL) or MINO@PLGA (1 mg/mL). Representative images of the scratch gap were captured at 0 h and 24 h. F Effect of MINO@PLGA on viability of HUVECs
This suggests that MINO@PLGA microspheres, with their sustained release and enhanced corneal penetration, contribute to prolonged drug contact with ocular tissues. Both MINO and MINO@PLGA exhibited inhibitory effects on HUVEC migration after 24 h, with MINO@PLGA demonstrating a slightly stronger effect (Fig. 2E, F). This indicates that encapsulated MINO retains its bioactivity, and the sustained release from PLGA microspheres may enhance its inhibitory effects, making it a potential superior agent for wound healing or anti-angiogenic applications.
The severity of corneal alkali burns and the extent of tissue damage can significantly impact the timing and intensity of the inflammatory response. In certain instances, inflammation and CoNV may persist for an extended duration, particularly when the injury is severe or complicated by issues such as infection. The majority of current treatments exhibit optimal efficacy within a relatively limited temporal window following the initiation of CoNV, with diminishing effectiveness as neovascularization matures and stabilizes [2]. The BEV@MSN nanoparticles, as prepared by Lyu et al. [9], exhibited a release duration of up to four weeks, with only about 27% and 33% released on the first and second days, respectively, followed by a nearly linear release over the subsequent three weeks. In contrast, the PLGA-bevacizumab nanoparticles prepared by Zhang et al. [22] demonstrated a different release profile, with more than 40% of bevacizumab being released within the first 2 h, and an additional approximate 40% released in the subsequent 7 days, followed by a slower release extending up to 21 days. MINO@PLGA extends the half-life of MINO and achieves sustained release from PLGA nanoparticles in a stable, controlled manner. This sustained release has the potential to continuously counteract the activity of corneal neovascularization. MINO@PLGA retains more effectively in the cornea, prolonging drug contact time with ocular tissues, delivering drugs to a specific tissue site in a controlled manner, protecting drugs from degradation. This may be attributed to the good water solubility of the MINO@PLGA microspheres and the superior liposolubility of MINO within the tetracycline category, enabling it to penetrate the corneal epithelium and reach the corneal stroma [24].
Abnormal endothelial cell proliferation, migration, and tube formation is critical for angiogenic effects [25]. MINO@PLGA might exhibit a stronger inhibitory effect on HUVECs migration, potentially making it a superior agent for wound healing or anti-angiogenic applications [26]. In addition, inadequate corneal penetration and the rapid clearance of drugs from the ocular surface significantly undermine the therapeutic efficacy of topical eye drops for the treatment of ocular diseases. Consequently, frequent administration of topical drugs or high-dose systemic medication is often required (30–60 mg/kg MINO daily) [15], which may result in reduced patient compliance and compromised therapeutic effectiveness. In our study, the dose and frequency of drug administration could be decreased.
Evaluation of anti-CoNV effect
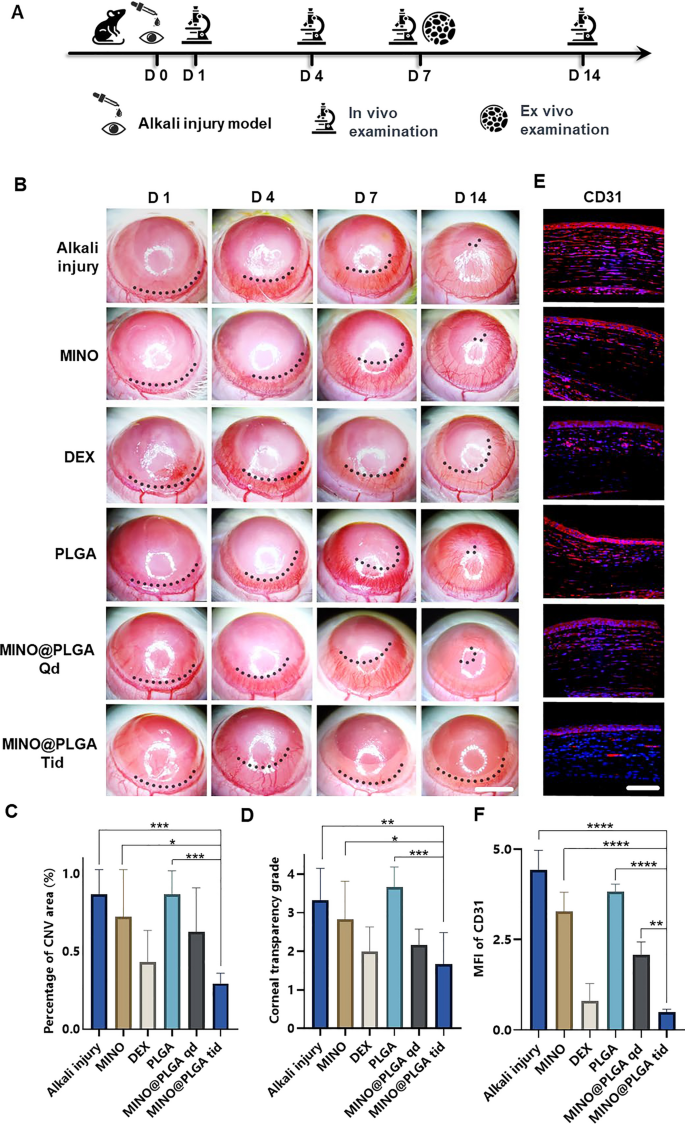
The corneal alkali burn model was established on day 0, followed by continuous observation for 14 days post-operation. Slit-lamp in vivo examinations were conducted on days 1, 4, 7, and 14, with a subset of rats sacrificed for ex vivo analysis on day 7 (Fig. 3A). The inhibitory effect of MINO@PLGA on CoNV was evaluated by continuously observing and comparing the CoNV area percentage and corneal transparency at the same corneal location between the MINO@PLGA (Qd and Tid) groups and the control group. From day 1 and 4 post-alkali burn, the neovascular buds in the MINO@PLGA (Qd and Tid) groups showed similarity to those in the control group (p > 0.05). From day 4 to day 14, corneal neovascularization in the group treated with MINO@PLGA Tid exhibited a decrease. In contrast, CoNV growth in the control group was notably robust, and it is time-dependent (Fig. 3B). The neovascular area ratio was further quantified. As shown in Fig. 3C, on day 14, the CoNV area for the MINO@PLGA Tid group was the smallest at 29.40% ± 6.55%. These values were lower than those in the control group (86.81% ± 15.71%), MINO group (72.42% ± 30.15%), PLGA group (86.87% ± 14.94%) (p < 0.05), and the DEX group (43.23% ± 20.35%), MINO@PLGA Qd group (62.78% ± 28.12%) (p > 0.05). Quantification confirmed the visual observations; the reduction of CoNV in corneas treated with MINO@PLGA suggests its potential in effectively managing post-injury corneal neovascularization. Corneal transparency, reflecting the edematous state, injury, and inflammatory condition, varied among treatments. MINO@PLGA (Qd and Tid) treatments led to decreased opacity (p < 0.05), suggesting improved wound healing and attenuation of inflammation crucial for maintaining corneal clarity (Fig. 3D). Samples treated with MINO@PLGA (Qd and Tid) exhibited decreased CD31 fluorescence, indicating reduced vascular formation (Fig. 3E). Compared to other samples (except for the DEX group), those treated with MINO@PLGA, especially Tid, demonstrated a notably lower MFI of CD31 (Fig. 3F) (p < 0.05).
MINO@PLGA inhibits corneal neovascularization and reduces haze. A Examination time flow after corneal alkali burn. B The corneal neovascularization was examined at 1, 4, 7, 14 days after the corneal alkali burn (scale bar: 3 mm), and the quantified corneal neovascularization of 14 days is shown in (C) (n = 6). D Corneal opacity at 14 days between groups (n = 6). E CD31 immunofluorescence at 7 days. F MFI of CD31 (scale bar: 100 μm, n = 3). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Minocycline, a tetracycline derivative, possesses the ability to penetrate the blood–brain barrier and serves as a broad-spectrum antibiotic with demonstrated anti-inflammatory, anti-apoptotic, and antioxidant properties [27]. Its extensive usage includes the treatment of respiratory infections, reproductive tract infections, skin infections, and suppurative infections [28]. Our previous study demonstrated minocycline has neuroprotective effect via its anti-inflammation mechanism [7]. Furthering the understanding of minocycline’s therapeutic effects, research by Yuan et al. [29] revealed its significant anti-inflammatory activity in rat models of endotoxin-induced uveitis and retinal inflammation. This was achieved primarily through the attenuation of LPS-induced expression of IL-1β and CCL-2. Previous research [15] involving systemic administration of minocycline, particularly through intraperitoneal injection in a mouse model of alkali burn-induced corneal neovascularization, demonstrated a decrease in CoNV to 73.03% ± 17.81%, in stark contrast to 97.43% ± 3.91% observed in the PBS group on day 14. In our study, we observed a markedly more significant reduction in the CoNV area with the MINO@PLGA treatment—specifically, 29.40% ± 6.55% compared to 86.81% ± 15.71% in the control group on day 14. This finding not only underscores the effectiveness of our MINO@PLGA treatment in reducing CoNV but also extends previous observations by showing that minocycline, when delivered effectively, can substantially decrease the extent of CoNV. These results solidify minocycline’s potential as a valuable therapeutic agent in the treatment of CoNV.
The reduced opacity with MINO@PLGA treatment suggests improved wound healing and attenuation of inflammation, which is crucial for maintaining corneal clarity [30]. The lower MFI of CD31 in MINO@PLGA-treated samples further quantifies its anti-angiogenic properties [31], suggesting a potential similar to the anti-inflammatory drug dexamethasone in managing corneal neovascular diseases, which was superior to MINO alone [32]. And Compared to the MINO@PLGA Qd group, the MINO@PLGA Tid group demonstrates the ability to maintain a sufficient drug concentration, thus improving efficacy in the treatment of CoNV and reducing opacity.
Injury healing
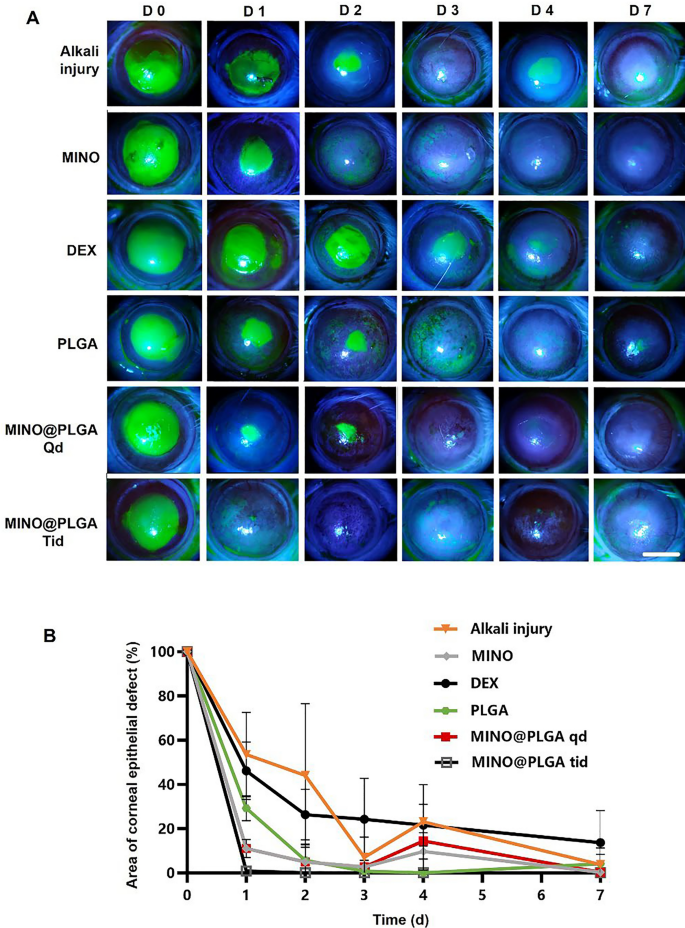
Fluorescein sodium staining was employed to visualize the progression of corneal epithelial injuries across all groups (Fig. 4A). Notably, both the MINO group and the MINO@PLGA group exhibited no signs of nonhealing in corneal epithelial injury repair, highlighting the efficacy of MINO and MINO@PLGA in promoting damage repair and controlling inflammation. Figure 4B illustrates the comparative analysis of epithelial healing rates among different treatment groups. MINO@PLGA (Qd and Tid) demonstrated a notably faster wound healing rate and better epithelial function recovery compared to other treatments, including the DEX group. The MINO@PLGA Tid group, in particular, exhibited efficient promotion of epithelial healing post-alkali burn. Corneas treated with MINO@PLGA Tid demonstrated rapid reductions in epithelial defect areas, with almost complete healing observed by day 7. In contrast, the Alkali injury group and the DEX group showed slower corneal epithelial healing and longer healing times (p < 0.05).
Changes in the corneal epithelial injury area over time in different groups after alkali burn. A Typical fluorescein sodium staining of the corneal epithelium was examined at 0, 1, 2,3, 4, 7 days after the corneal alkali burn (scale bar: 3 mm), the percentage of corneal epithelial defect area over time is shown in (B) (n = 6)
Corneal keratolysis, often associated with heightened inflammation and impaired re-epithelialization, necessitates strategies that minimize inflammation and expedite re-epithelization to prevent complications [33, 34]. It is known that glucocorticoids, such as dexamethasone (DEX), can impede wound healing by hindering cell migration, as demonstrated in previous studies [35, 36]. Our findings align with this notion, showing that the DEX group exhibited delayed corneal epithelial healing compared to other treatments.
In contrast, both the MINO group and the MINO@PLGA group displayed efficient corneal epithelial injury repair, underscoring the advantages of MINO and MINO@PLGA in promoting damage repair while effectively controlling inflammation. Fluorescein sodium staining confirmed the progress of corneal epithelial injuries, providing a visual representation of the positive outcomes associated with MINO and MINO@PLGA treatments.
The results from Fig. 4B further emphasize the superiority of MINO@PLGA (Qd and Tid) in promoting epithelial healing compared to other treatments, including the DEX group. The faster recovery observed in the MINO@PLGA Tid group suggests that frequent application of this formulation may offer superior treatment effects in promoting wound healing after alkali injury. This aligns with the crucial role of accelerated re-epithelialization in advancing overall wound healing [37]. These findings collectively highlight the potential clinical significance of MINO@PLGA in managing acute ocular alkali burns by facilitating prompt corneal epithelial healing while minimizing inflammation-related complications.
Immunofluorescent staining
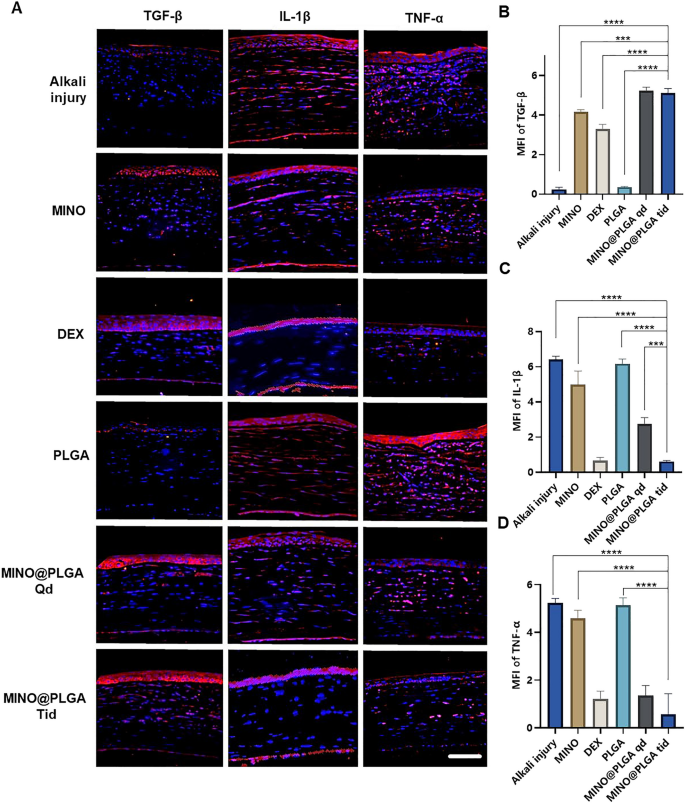
Images reveal different levels of corneal TGF-β and inflammatory factors IL-1β and TNF-α in various treatment groups on day 7 post-alkali burn. In the control group, the expression level of TGF-β in the corneal epithelium is decreased, while levels of IL-1β and TNF-α in the corneal stroma are elevated. With dexamethasone, MINO, and MINO@PLGA treatments, there is an increased expression of TGF-β, while inflammatory factors IL-1β and TNF-α show varying degrees of decrease (Fig. 5A). Semi-quantitative analysis results (Fig. 5B) indicate that the expression of TGF-β was highest in the MINO@PLGA Qd and MINO@PLGA Tid groups (5.23 ± 0.18, 5.12 ± 0.23 respectively), followed by the MIMO group (4.16 ± 0.12). The Dexamethasone group showed a slight decrease (3.23 ± 0.25), while the Alkali injury and PLGA groups exhibited the lowest values (0.24 ± 0.10 and 0.35 ± 0.03 respectively). The expression of IL-1β and TNF-α was lowest in the MINO@PLGA Tid group (0.61 ± 0.04 and 0.57 ± 0.50, respectively) and showed statistically significant differences when compared to the control group (p < 0.0001) (Fig. 5C, D). The reduction of inflammatory in the cornea was also evidenced by H&E staining of corneal tissue (Additional file 1: Fig. S1). This suggests that MINO@PLGA might suppress CoNV through the downregulation of inflammatory factors IL-1β and TNF-α via the TGF-β pathway.
During alkali burns, the corneal epithelium sustains damage, resulting in a deficiency of pluripotent limbal stem cells and an imbalance between angiogenic stimulators and inhibitors. This imbalance can lead to the over-proliferation and migration of capillary endothelial cells into the damaged cornea [38]. The corneal epithelium plays a pivotal role in the mechanism of neovascularization. The expression of inflammatory factors induced by TGF-β exerts anti-neovascular effects [39]. TGF-β has also been reported to stimulate the transdifferentiation of corneal stromal cells into myofibroblasts, thereby increasing corneal opacity [40]. Pruzanski et al. [41] demonstrated that minocycline inhibits phospholipase A2 (PLA2) and interacts with the substrate to attenuate the inflammatory response in patients with rheumatoid arthritis. In the context of inflammation, glial cells, both astrocytes and microglia, play pivotal roles [42]. Recent studies have shown that minocycline mitigates the development of pain hypersensitivity by inhibiting microglial activation, reducing proinflammatory cytokine expression in both inflammatory and neuropathic pain, and downregulating the production of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [43]. Furthermore, minocycline was found to inhibit p38 MAPK in microglia, specifically targeting this pathway. P38 MAPK is known to mediate inflammatory responses in various cell types, including microglia.
With the progressive elucidation of minocycline’s anti-inflammatory effects and underlying mechanisms, numerous studies have demonstrated its multifaceted properties in various animal models of neuronal degenerative diseases. Minocycline exhibits antimicrobial, anti-inflammatory, anti-apoptotic, and neuroprotective characteristics in conditions such as Parkinson’s disease, multiple sclerosis, Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and retinitis pigmentosa [44, 45]. Scholz et al. [46] demonstrated that minocycline provides protection against retinal damage in a mouse model of acute retinal degeneration induced by white-light irradiation. Administration of minocycline led to the inhibition of inflammatory factors, including CCL2, IL-6, and iNOS, and resulted in the suppression of pro-inflammatory responses and neurotoxicity in microglia, while promoting the survival of photoreceptors. In this study, we have demonstrated, for the first time, that minocycline, via the TGF-β pathway, downregulates the inflammatory factors IL-1β and TNF-α to effectively suppress CoNV.
Biocompatibility
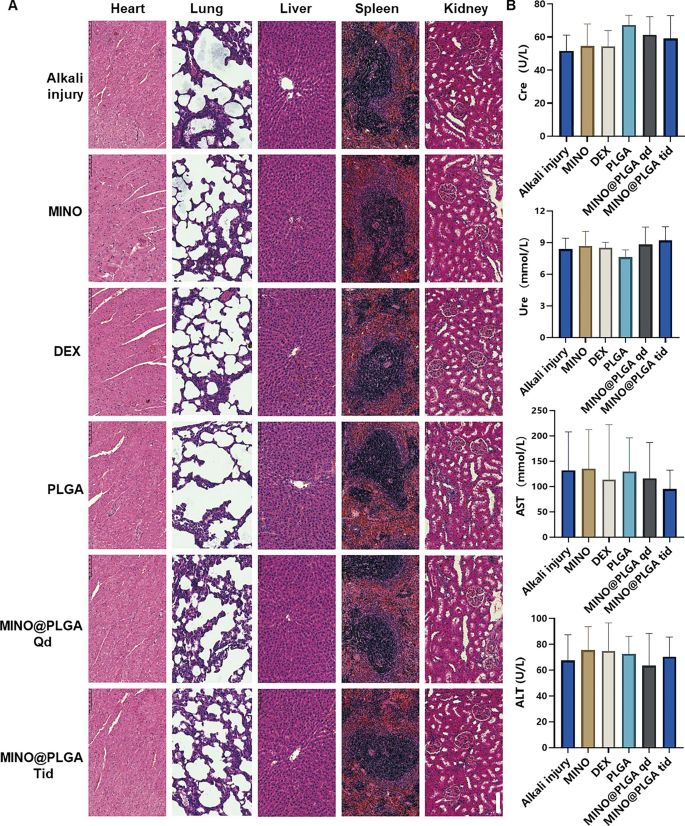
To assess the biosafety of MINO@PLGA, histological examination of major organ tissues (Heart, lung, liver, spleen, kidney) was performed using H&E staining (Fig. 6A). The images across all treatment groups, including MINO@PLGA treatments, revealed no overt signs of organ damage. This suggests a favorable safety profile for MINO@PLGA, with no discernible pathological changes in major organs following its use post-alkali burn. Biochemical parameters from blood samples, including Creatinine (Cre), Urea (Ure), Alanine aminotransferase (ALT), and Aspartate aminotransferase (AST), were analyzed to further evaluate the safety of MINO@PLGA (Fig. 6B). The data indicated that these parameters remained within normal reference ranges across all treatment groups. The maintenance of normal levels suggests that MINO@PLGA does not adversely impact renal or liver function, reinforcing its safety as a treatment option following alkali burns.
Evaluation of MINO@PLGA safety on organ histopathology and systemic blood parameters. A H&E of major organs in rats from different groups at 7 days after corneal alkali burn (scale bar: 100 μm). B Biochemical indicators in the orbital venous blood of rats from different groups at 7 days after alkali burn. Creatinine (Cre) reference range: 19.43–64.97 mmol/L. Urea (Ure) reference range: 2.08–7.75 mmol/L. Alanine aminotransferase (ALT) reference range: 33.7–98.7 U/L. Aspartate aminotransferase (AST) reference range: 69.7–322.9 U/L (n = 6)
The systemic administration of minocycline is associated with various common adverse side effects [47]. Serious adverse effects include hypersensitivity syndrome reaction, drug-induced lupus, idiopathic intracranial hypertension, and other autoimmune syndromes, which may lead to fatal outcomes [43]. To enhance therapeutic efficacy and achieve highly localized effects while minimizing the risk of side effects compared to systemic administration, the use of locally delivered drug-loaded biodegradable nanoparticles presents an appealing alternative [6]. Topical administration, necessitating a significantly lower dosage than systemic routes, is favored for ophthalmic drug delivery [48]. Local injections, such as subconjunctival or intravitreal, are invasive and thus reserved for administration by healthcare professionals. Our study emphasized the topical administration of eye drops, a patient-friendly method that can be self-administered [49].