Nanoparticles subdue antibiotic-resistant bacteria’s defences while enhancing innate immunity
A method for overcoming antibiotic resistance uses multimodal nanoparticles that target bacterial defence mechanisms while enhancing the innate immune response.
The rise in antibiotic resistance is considered a slow-moving medical catastrophe, as these revolutionary drugs that have kept us relatively safe from bacterial infection for decades are losing their efficacy. In part due to their co-evolution, bacterial pathogens have developed mechanisms to resist almost every antibiotic on the market and we are in desperate need for new, innovative approaches. Writing in Nature Nanotechnology, Zhu et al. present a nanoparticle-based possibility, in which they target bacterial defence mechanisms while simultaneously enhancing the ability of the host immune cells to fight infection1.
Staphylococcus aureus, the focus pathogen in this work, is a formidable opponent. Methicillin-resistant S. aureus (MRSA) is the leading cause of death by drug-resistant bacteria in the developed world2. It causes a variety of infections ranging from minor skin and soft tissue infections, through invasive bloodstream, osteomyelitis and necrotizing pneumonia infections3. S. aureus has an extensive arsenal of virulence factors at its disposal and thrives in multiple host niches. The breadth of its arsenal means it has a level of redundancy in its virulence and several compensatory mechanisms. Vaccine development and immunotherapeutic activation of the adaptive immune system against S. aureus has been complicated partially due to the fact it also asymptomatically colonizes a substantial proportion of the population3. Activating the adaptive immune system against a colonizer may lead to enduring inflammation and other unwanted side effects.
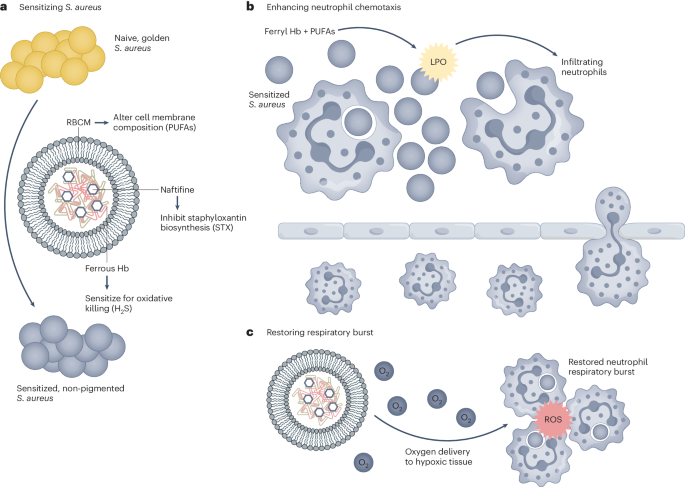
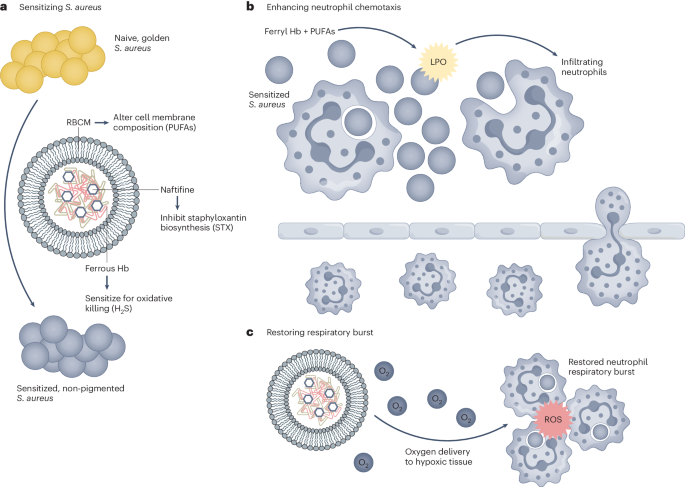
Zhu et al. use a multidimensional approach to overcome some of the previous shortcomings of S. aureus immunotherapy (Fig. 1). Using a self-assembling nanoparticle (NP) methodology, the authors aim to both attenuate bacterial defence systems while also enhancing the activity of innate host immune cells. The work focusses on the activity of neutrophils, a key early responder to S. aureus. As the innate immune system lacks immune memory, one of the main causes of enduring side effects, targeting these cells during ongoing infection is a promising goal. The most effective NPs described in the work are made up of a red blood cell membrane (RBCM) coating covering a self-assembling core consisting of naftifine, an FDA-approved repurposed antifungal agent, and haemoglobin, the protein in red blood cells that carries oxygen throughout the body. The use of biocompatible, repurposed and already approved elements enhances the translatability of this work.
Zhu et al. use a multifaceted approach to combat S. aureus infection. a–c, They use multi-component nanoparticles to sensitize S. aureus (a), enhance neutrophil chemotaxis (b) and enhance the activity of recruited neutrophils (c). LPO, lipid peroxide. Adapted from ref. 1, Springer Nature Ltd.
The RBCM coating is shown to not only be a biocompatible outer membrane on the NPs but it alters the incorporation of polyunsaturated fatty acids into the bacterial cell membrane, inducing lipid peroxidation, both sensitizing the bacteria to neutrophil killing and promoting neutrophil chemotaxis. Naftifine inhibits the ability of S. aureus to synthesize the antioxidant staphyloxanthin (STX), the carotenoid pigment that gives S. aureus its distinctive golden hue. Suppression of STX sensitizes S. aureus to neutrophil killing and has been previously suggested as a promising target for S. aureus treatment4,5. Oxygen-carrying haemoglobin in the NPs reduces bacterial hydrogen sulfide levels, re-sensitizing them to host oxidant killing while also reducing hypoxia in the infectious environment and subsequently enhancing neutrophil oxidative burst.
The authors demonstrate extensive evaluation of this approach both in vitro and in vivo. They show evidence for each element of the NP composition and show efficacy in several host environments. A combination of the NPs with neutrophils is consistently shown to be most effective for bacterial killing. The NP–neutrophil combination is shown to be effective against not only planktonic bacteria but also models of persister cells, a clinically challenging subset of metabolically inactive bacteria, as well as bacterial biofilms, where bacteria are embedded in a protective extracellular matrix. Of note is that the authors show that the designed NPs increase the efficacy of both murine and human neutrophil killing towards S. aureus. S. aureus has a well-defined, yet subtle human specificity to its pathogenicity and this has been particularly shown in its interactions with neutrophils6. Demonstrating efficacy in both these species strengthens the clinical potential of this approach. The successful NP treatment is demonstrated in multiple in vivo infection models of thigh and lung infection, peritonitis and bacteriemia. The data from the animal models is exceptional, with greatly reduced bacteria loads, increased neutrophil recruitment and a complete rescue of treated animals in a fatal bacteriemia model. While these models could be further refined to more closely model clinical situations, the data shown appear very promising.
An interesting element of this work is how the authors use a nanomedical approach to not only target bacterial virulence but to alter the tissue microenvironment. Hypoxia, or a loss of adequate oxygen supply, occurs in numerous infectious environments and reduces the efficacy of neutrophil killing while also causing local tissue damage7. The reduction of hypoxia shown here may not only increase the neutrophil killing efficacy but could also reduce collateral tissue damage, though this remains to be investigated. As hypoxia is a hallmark of multiple infections this approach may also be readily adaptable to other problematic infections.
Immunotherapeutic approaches to bacterial infection face numerous challenges. A pathogen such as S. aureus is particularly perplexing as it has extensive immune evasion mechanisms and the adaptive immune defence mechanisms to this pathogen are ineffective. By taking a nanomedical approach, Zhu et al. demonstrate the potential of a nanoparticle-based combination therapy that targets both bacterial virulence and enhances innate immunity. While a great deal of work is still needed to bring these developments to clinical use, it demonstrates an innovative multiplexed approach using biological and repurposed elements. MRSA has repeatedly shown its capability of developing resistance to antimicrobials targeting a single element or virulence factor, multipronged approaches as described here are going to be much needed as the antimicrobial resistance crisis continues.