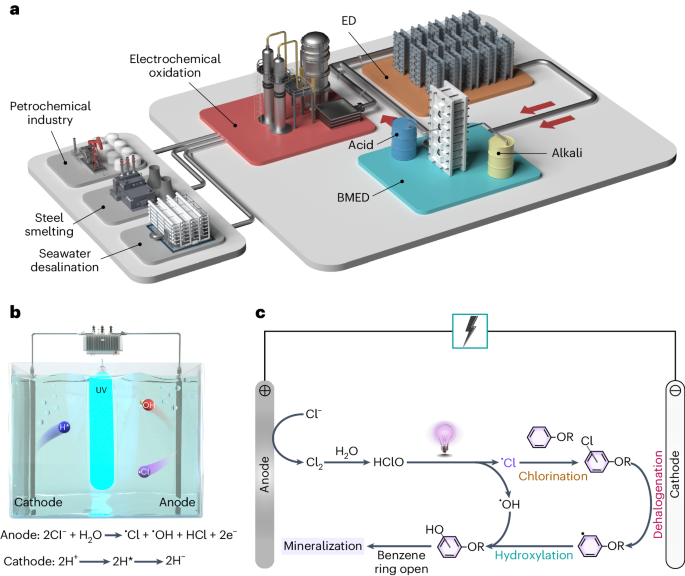
Redox-neutral electrochemical decontamination of hypersaline wastewater with high technology readiness level
Chung, M. G., Frank, K. A., Pokhrel, Y., Dietz, T. & Liu, J. Natural infrastructure in sustaining global urban freshwater ecosystem services. Nat. Sustain. 4, 1068–1075 (2021).
Zhang, X. et al. Managing nitrogen for sustainable development. Nature 528, 51–59 (2015).
Panagopoulos, A. & Giannika, V. Comparative techno-economic and environmental analysis of minimal liquid discharge (MLD) and zero liquid discharge (ZLD) desalination systems for seawater brine treatment and valorization. Sustain. Energy Technol. Assess. 53, 102477 (2022).
Menon, A. K., Haechler, I., Kaur, S., Lubner, S. & Prasher, R. S. Enhanced solar evaporation using a photo-thermal umbrella for wastewater management. Nat. Sustain. 3, 144–151 (2020).
Kumar, A., Phillips, K. R., Thiel, G. P., Schröder, U. & Lienhard, J. H. Direct electrosynthesis of sodium hydroxide and hydrochloric acid from brine streams. Nat. Catal. 2, 106–113 (2019).
Shehzad, M. A. et al. Shielded goethite catalyst that enables fast water dissociation in bipolar membranes. Nat. Commun. 12, 9 (2021).
Zhang, W. et al. Impact of chloride ions on UV/H2O2 and UV/persulfate advanced oxidation processes. Environ. Sci. Technol. 52, 7380–7389 (2018).
Chen, Y. et al. Tip-intensified interfacial microenvironment reconstruction promotes an electrocatalytic chlorine evolution reaction. ACS Catal. 12, 14376–14386 (2022).
Liu, J., Zhang, X. & Li, Y. Photoconversion of chlorinated saline wastewater DBPs in receiving seawater is overall a detoxification process. Environ. Sci. Technol. 51, 58–67 (2017).
Yang, T. et al. Efficient degradation of organoarsenic by UV/chlorine treatment: kinetics, mechanism, enhanced arsenic removal, and cytotoxicity. Environ. Sci. Technol. 55, 2037–2047 (2021).
Rao, U. et al. Structural dependence of reductive defluorination of linear PFAS compounds in a UV/electrochemical system. Environ. Sci. Technol. 54, 10668–10677 (2020).
Guo, K., Wu, Z., Chen, C. & Fang, J. UV/chlorine process: an efficient advanced oxidation process with multiple radicals and functions in water treatment. Acc. Chem. Res. 55, 286–297 (2022).
Choi, C. et al. Efficient electrocatalytic valorization of chlorinated organic water pollutant to ethylene. Nat. Nanotechnol. 18, 160–167 (2023).
Fang, J., Fu, Y. & Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 48, 1859–1868 (2014).
Zhang, J., Zhang, G., Lan, H., Qu, J. & Liu, H. Synergetic hydroxyl radical oxidation with atomic hydrogen reduction lowers the organochlorine conversion barrier and potentiates effective contaminant mineralization. Environ. Sci. Technol. 55, 3296–3304 (2021).
Llorente, M. J., Nguyen, B. H., Kubiak, C. P. & Moeller, K. D. Paired electrolysis in the simultaneous production of synthetic intermediates and substrates. J. Am. Chem. Soc. 138, 15110–15113 (2016).
Min, Y. et al. Mimicking reductive dehalogenases for efficient electrocatalytic water dechlorination. Nat. Commun. 14, 5134 (2023).
Dwinandha, D., Zhang, B. & Fujii, M. Prediction of reaction mechanism for OH radical-mediated phenol oxidation using quantum chemical calculation. Chemosphere 291, 132763 (2022).
Atobe, M., Tateno, H. & Matsumura, Y. Applications of flow microreactors in electrosynthetic processes. Chem. Rev. 118, 4541–4572 (2018).
Finke, C. E. et al. Enhancing the activity of oxygen-evolution and chlorine-evolution electro-catalysts by atomic layer deposition of TiO2. Energy Environ. Sci. 12, 358–365 (2019).
Karlsson, R. K. B. & Cornell, A. Selectivity between oxygen and chlorine evolution in the chlor-alkali and chlorate processes. Chem. Rev. 116, 2982–3028 (2016).
Näslund, L.-Å. et al. The role of TiO2 doping on RuO2-coated electrodes for the water oxidation reaction. J. Phys. Chem. C 117, 6126–6135 (2013).
Jiao, Y., Jiang, H. & Chen, F. RuO2/TiO2/Pt ternary photocatalysts with epitaxial heterojunction and their application in CO oxidation. ACS Catal. 4, 2249–2257 (2014).
Morales-Guio, C. G. et al. Solar hydrogen production by amorphous silicon photocathodes coated with a magnetron sputter deposited Mo2C catalyst. J. Am. Chem. Soc. 137, 7035–7038 (2015).
Dong, C. et al. Fully exposed palladium cluster catalysts enable hydrogen production from nitrogen heterocycles. Nat. Catal. 5, 485–493 (2022).
Xiong, H. et al. Engineering catalyst supports to stabilize PdOx two-dimensional rafts for water-tolerant methane oxidation. Nat. Catal. 4, 830–839 (2021).
Ghassemzadeh, L., Peckham, T. J., Weissbach, T., Luo, X. & Holdcroft, S. Selective formation of hydrogen and hydroxyl radicals by electron beam irradiation and their reactivity with perfluorosulfonated acid ionomer. J. Am. Chem. Soc. 135, 15923–15932 (2013).
Xu, B., Chen, Z., Zhang, G. & Wang, Y. On-demand atomic hydrogen provision by exposing electron-rich cobalt sites in an open-framework structure toward superior electrocatalytic nitrate conversion to dinitrogen. Environ. Sci. Technol. 56, 614–623 (2022).
Wang, J. et al. Ambient ammonia synthesis via palladium-catalyzed electrohydrogenation of dinitrogen at low overpotential. Nat. Commun. 9, 1795 (2018).
Kakade, B. A., Tamaki, T., Ohashi, H. & Yamaguchi, T. Highly active bimetallic PdPt and CoPt nanocrystals for methanol electro-oxidation. J. Phys. Chem. C 116, 7464–7470 (2012).
Zuo, K. et al. Electrified water treatment: fundamentals and roles of electrode materials. Nat. Rev. Mater. 8, 472–490 (2023).
Wang, H.-X., Toh, W. L., Tang, B. Y. & Surendranath, Y. Metal surfaces catalyse polarization-dependent hydride transfer from H2. Nat. Catal. 6, 351–362 (2023).
Sakamoto, H. et al. Photocatalytic dehalogenation of aromatic halides on Ta2O5-supported Pt–Pd bimetallic alloy nanoparticles activated by visible light. ACS Catal. 7, 5194–5201 (2017).
Walter, T. H. et al. Spin trapping in heterogeneous electron transfer processes. Can. J. Chem. 60, 1621–1636 (1982).
Barroso-Martínez, J. S. et al. Real-time detection of hydroxyl radical generated at operating electrodes via redox-active adduct formation using scanning electrochemical microscopy. J. Am. Chem. Soc. 144, 18896–18907 (2022).
Hong, J. et al. Metastable hexagonal close-packed palladium hydride in liquid cell TEM. Nature 603, 631–636 (2022).
Van Buren, J., Prasse, C., Marron, E. L., Skeel, B. & Sedlak, D. L. Ring-cleavage products produced during the initial phase of oxidative treatment of alkyl-substituted aromatic compounds. Environ. Sci. Technol. 54, 8352–8361 (2020).
Wang, Y., Wei, Y., Song, W., Chen, C. & Zhao, J. Photocatalytic hydrodehalogenation for the removal of halogenated aromatic contaminants. ChemCatChem 11, 258–268 (2019).
Yang, J. et al. CO2-mediated organocatalytic chlorine evolution under industrial conditions. Nature 617, 519–523 (2023).
Over, H. Surface chemistry of ruthenium dioxide in heterogeneous catalysis and electrocatalysis: from fundamental to applied research. Chem. Rev. 112, 3356–3426 (2012).
Lin, J. et al. Shielding effect enables fast ion transfer through nanoporous membrane for highly energy-efficient electrodialysis. Nat. Water 1, 725–735 (2023).
Butler, J. D., Parkerton, T. F., Redman, A. D., Letinski, D. J. & Cooper, K. R. Assessing aromatic-hydrocarbon toxicity to fish early life stages using passive-dosing methods and target-lipid and chemical-activity models. Environ. Sci. Technol. 50, 8305–8315 (2016).
Stinn, C. & Allanore, A. Selective sulfidation of metal compounds. Nature 602, 78–83 (2022).
Zabaniotou, A. Redesigning a bioenergy sector in EU in the transition to circular waste-based bioeconomy—a multidisciplinary review. J. Clean. Prod. 177, 197–206 (2018).
Andrew, R. M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 10, 195–217 (2018).
Zhao, C., Dao, R., Wang, Y., Yao, J. & Li, H. A DFT investigation exploring the influence of lone electron pair on hyperfine structures of N-centered radicals. Chem. Phys. 517, 13–23 (2019).
Houriez, C., Ferre, N., Siri, D., Tordo, P. & Masella, M. Assessing the accuracy of a QM/MM//MD combined protocol to compute spectromagnetic properties of polyfunctional nitroxides in solution. Theor. Chem. Acc. 131, 1240 (2012).
Yamaguchi, M. DFT calculation of isotropic hyperfine coupling constants of spin adducts of 5,5-dimethyl-1-pyrroline-N-oxide in benzene and water. Comput. Theor. Chem. 1104, 24–31 (2017).
Cohen, A. J., Mori-Sánchez, P. & Yang, W. Challenges for density functional theory. Chem. Rev. 112, 289–320 (2012).
Gaussian 16 rev. C.01 (Gaussian, Inc., 2016).
Zhang, G. & Liu, H. Source data for “Redox-neutral electrochemical decontamination of hypersaline wastewater with high technology readiness level”. figshare https://doi.org/10.6084/m9.figshare.25390759 (2024).