Team describes MXene-supported PtCo bimetallic catalyst for hydrogen evolution in acidic conditions
Hydrogen energy is considered a promising solution with high energy density and zero pollution emissions. Currently, hydrogen is mainly derived from fossil fuels, which increases energy consumption and greenhouse gas emissions, hindering efforts to achieve carbon neutrality goals.
Electrochemical water splitting using renewable energy is an environmentally sustainable method for hydrogen production. To improve hydrogen production efficiency and reduce energy consumption, it is necessary to find efficient hydrogen evolution reaction (HER) catalysts.
Platinum (Pt) group metals are commonly used as HER catalysts due to their excellent natural activity. However, the scarcity and high cost of these resources mean that they limited widespread application. Increasing metal atom utilization to develop low-loading Pt catalysts is crucial. Recently, supported catalysts have been considered an effective approach to minimize the amount of precious metal loading and maintain their excellent activity.
MXene materials—with their layered nanostructure, high conductivity, good hydrophilicity, and rich surface chemical properties—have found wide applications in catalysis.
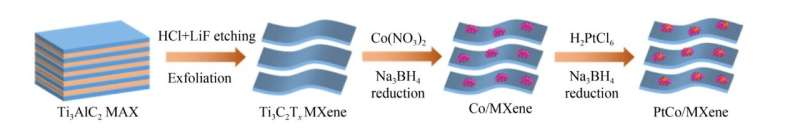
A research group including Kai-Ling Zhou, Yang Yang, Yuhong Jin, and Hao Wang from the Beijing University of Technology and Institute of Deep-Sea Science and Engineering, Chinese Academy of Sciences, has fabricated small and highly-dispersed PtCo bimetallic catalysts on MXene (PtCo/MXene) using a step-by-step reduction approach. They have studied the HER electrocatalytic activity of PtCo/MXene in an acidic medium.
The research is published in the journal Frontiers in Energy.
The team found that the introduction of Co species changed the electronic structure of the active site and promoted the catalytic performance of Pt precious metal in HER. The PtCo/MXene catalyst exhibits a superior HER activity with a low overpotential of 60 and 152 mV at current densities of −10 and −100 mA/cm2, respectively, and excellent working durability in the 0.5 mol/L H2SO4 medium.
The PtCo/MXene catalyst possesses a considerable specific surface area and minimal charge transfer impedance. The DFT calculation shows that PtCo bimetal can promote the desorption of H* and promote the HER process in an acidic medium.
This work provides a valuable perspective to introduce low-load precious metals on MXene and guarantee its activity and stability.
More information:
Guangxun Chen et al, MXene supported PtCo bimetallic catalyst for hydrogen evolution in acidic conditions, Frontiers in Energy (2024). DOI: 10.1007/s11708-024-0925-9
Provided by
Higher Education Press
Citation:
Team describes MXene-supported PtCo bimetallic catalyst for hydrogen evolution in acidic conditions (2024, May 6)
retrieved 6 May 2024
from https://phys.org/news/2024-05-team-mxene-ptco-bimetallic-catalyst.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

