| May 02, 2024 |
|
|
|
(Nanowerk News) Swedish researchers at Chalmers University of Technology and the University of Gothenburg have developed an AI method that improves the identification of toxic chemicals – based solely on knowledge of the molecular structure. The method can contribute to better control and understanding of the ever-growing number of chemicals used in society, and can also help reduce the amount of animal tests.
|
|
The study has been published in Science Advances (“Transformers enable accurate prediction of acute and chronic chemical toxicity in aquatic organisms”).
|
|
The use of chemicals in society is extensive, and they occur in everything from household products to industrial processes. Many chemicals reach our waterways and ecosystems, where they may cause negative effects on humans and other organisms. One example is PFAS, a group of problematic substances which has recently been found in concerning concentrations in both groundwater and drinking water. It has been used, for example, in firefighting foam and in many consumer products.
|
|
Negative effects for humans and the environment arise despite extensive chemical regulations, that often require time-consuming animal testing to demonstrate when chemicals can be considered as safe. In the EU alone, more than two million animals are used annually to comply with various regulations. At the same time, new chemicals are developed at a rapid pace, and it is a major challenge to determine which of these that need to be restricted due to their toxicity to humans or the environment.
|
Valuable help in the development of chemicals
|
|
The new method developed by the Swedish researchers utilises artificial intelligence for rapid and cost-effective assessment of chemical toxicity. It can therefore be used to identify toxic substances at an early phase and help reduce the need for animal testing.
|
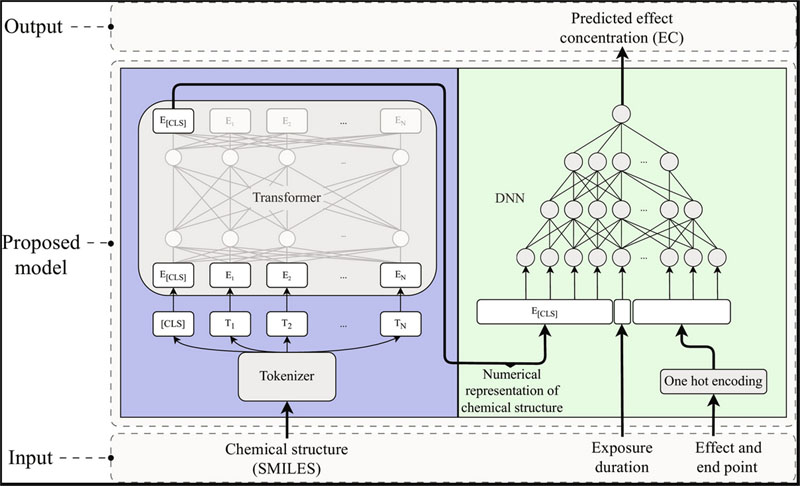 |
| Transformers enable accurate prediction of acute and chronic chemical toxicity in aquatic organisms. A representation of the molecule’s structure is used as input to a pretrained transformer, which interprets the molecular structure. The transformer creates a so-called “vector embedding” – a numerical representation of the toxicity of the structure. That is then used as input to a deep neural network (DNN), together with information about the type of toxic effect you want to assess and the exposure duration. The output of the neural network is the predicted molecule concentration that causes the requested effect. (Image: Chalmers University of Technology)
|
|
“Our method is able to predict whether a substance is toxic or not based on its chemical structure. It has been developed and refined by analysing large datasets from laboratory tests performed in the past. The method has thereby been trained to make accurate assessments for previously untested chemicals,” says Mikael Gustavsson, researcher at the Department of Mathematical Sciences at Chalmers University of Technology, and at the Department of Biology and Environmental Sciences at the University of Gothenburg.
|
|
“There are currently more than 100,000 chemicals on the market, but only a small part of these have a well-described toxicity towards humans or the environment. To assess the toxicity of all these chemicals using conventional methods, including animal testing, is not practically possible. Here, we see that our method can offer a new alternative,” says Erik Kristiansson, professor at the Department of Mathematical Sciences at Chalmers and at the University of Gothenburg.
|
|
The researchers believe that the method can be very useful within environmental research, as well as for authorities and companies that use or develop new chemicals. They have therefore made it open and publicly available.
|
Broader and more accurate than today’s computational tools
|
|
Computational tools for finding toxic chemicals already exist, but so far, they have had too narrow applicability domains or too low accuracy to replace laboratory tests to any greater extent. In the researchers’ study, they compared their method with three other, commonly used, computational tools, and found that the new method has both a higher accuracy and that it is more generally applicable.
|
|
“The type of AI we use is based on advanced deep learning methods,” says Erik Kristiansson. “Our results show that AI-based methods are already on par with conventional computational approaches, and as the amount of available data continues to increase, we expect AI methods to improve further. Thus, we believe that AI has the potential to markedly improve computational assessment of chemical toxicity.”
|
|
The researchers predict that AI systems will be able to replace laboratory tests to an increasingly greater extent.
|
|
“This would mean that the number of animal experiments could be reduced, as well as the economic costs when developing new chemicals. The possibility to rapidly prescreen large and diverse bodies of data can therefore aid the development of new and safer chemicals and help find substitutes for toxic substances that are currently in use. We thus believe that AI-based methods will help reduce the negative impacts of chemical pollution on humans and on ecosystem services,” says Erik Kristiansson.
|
More about: the new AI method
|
|
The method is based on transformers, an AI model for deep learning that was originally developed for language processing. Chat GPT – whose abbreviation means Generative Pretrained Transformer – is one example of the applications.
|
|
The model has recently also proved highly efficient at capturing information from chemical structures. Transformers can identify properties in the structure of molecules that cause toxicity, in a more sophisticated way than has been previously possible.
|
|
Using this information, the toxicity of the molecule can then be predicted by a deep neural network. Neural networks and transformers belong to the type of AI that continuously improves itself by using training data – in this case, large amounts of data from previous laboratory tests of the effects of thousands of different chemicals on various animals and plants.
|